
Science - Class 9

Is Matter Around Us Pure - Questions
Q67. How can we separate a mixture of salt and ammonium chloride?
Q68. Why alloy is considered as a mixture?
Or
Why is an alloy a mixture?
Q69. Write the principle and applications of separating funnel.
Q70. (a) Under which category of mixtures will you classify alloys and why?
(b) A solution is always a liquid. Comment.
(c) Can a solution be heterogeneous?
Q71. What is crystallization? Why is crystallization better than evaporation?
Q72. What are the components of a Colloidal Solution?
Q73. How can we obtain different gases from air?
Q74. What is a concentration of a solution?
Q75. You are provided with a mixture containing sand, iron filings, ammonium chloride and sodium chloride. Describe the procedures you would use to separate these constituents from the mixture?
Q76. A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light. They placed a milk sample contained in a beaker/tumbler in the box as shown in the Figure. They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it?
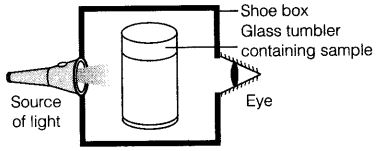
(a) Explain why the milk sample was illuminated? Name the phenomenon involved.
(b) Same results were not observed with a salt solution. Explain.
(c) Can you suggest two more solutions which would show the same effect as shown by the milk solution?
Q77. Differentiate between elements and compounds.
Q78. Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle