Science - Class 9

Is Matter Around Us Pure - Questions
Q1. Which of the following statements are true for pure substances?
(i) Pure substances contain only one kind of particles.
(ii) Pure substances may be compounds or mixtures.
(iii) Pure substances have the same composition throughout.
(iv) Pure substances can be exemplified by all elements other than nickel.
(a) (i) and (ii)
(b) (i) and (iii)
(c) (iii) and (iv)
(d) (ii) and (iii)
Q2. Rusting of an article made up of iron is called
(a) corrosion and it is a physical as well as chemical change
(b) dissolution and it is a physical change
(c) corrosion and it is a chemical change
(d) dissolution and it is a chemical change.
Q3. A mixture of sulphur and carbon disulphide is
(a) heterogeneous and shows Tyndall effect
(b) homogeneous and shows Tyndall effect
(c) heterogeneous and does not show Tyndall effect
(d) homogeneous and does not show Tyndall effect.
Q4. Tincture of iodine has antiseptic properties. This solution is made by dissolving
(a) iodine in potassium iodide
(b) iodine in vaseline
(c) iodine in water
(d) iodine in alcohol.
Q5. Which of the following are homogeneous in nature?
(i) Ice
(ii) Wood
(iii) Soil
(iv) Air
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv)
Q6. Which of the following are physical changes?
(i) Melting of iron metal
(ii) Rusting of iron
(iii) Bending of an iron rod :
(iv) Drawing a wire of iron metal
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (i), (iii) and (iv)
(d) (ii), (iii) and (iv)
Q7. Which of the following are chemical changes?
(i) Decaying of wood
(ii) Burning of wood
(iii) Sawing of wood
(iv) Hammering of a nail into a piece of wood
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Q8. Two substances, A and B were made to react to form a third substance, A2B according to the following reaction: 2A + B → A2B
Which of the following statements concerning this reaction are incorrect?
(i) The product A2B shows the properties of substances A and B.
(ii) The product will always have a fixed composition.
(iii) The product so formed cannot be classified as a compound.
(iv) The product so formed is an element.
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (iv)
(c) (i), (iii) and (iv)
(d) (iii) and (iv)
Q9. Two chemical species X and Y combine together to form a product P which contains both X and Y
X+Y→ P
X and Y cannot be broken down into simpler substances by simple chemical reactions. Which of the following concerning the species X, Y and P are correct?
(i) P is a compound.
(ii) X and Y are compound.
(iii) X and Y are elements.
(iv) P has a fixed composition.
(a) (i), (ii) and (iii),
(b) (i), (ii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (iii) and (iv)
Q10. Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution.
Q11. Salt can be recovered from its solution by evaporation. Suggest some other technique for the same?
Q12. What is pure substance?
Q13. What are the constituents of brass?
Or
Name the constituents elements of the alloy Brass.
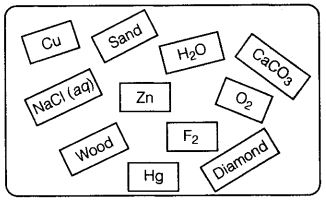
Q14. Classify the substances given in Fig. into elements and compounds.