
Science - Class 9

Topic outline
-
NCERT Solutions for Class 9 Science- Is Matter Around Us Pure (Chemistry), NCERT Textbook Solutions for Class 9 Science, NCERT Solutions For Class 9 Chemistry, Is Matter Around Us Pure - Class 9th NCERT Solutions Science, NCERT Solutions For Class 9 Chemistry Science Chapter 2 - Is Matter Around Us Pure, Science - Chemistry - Class 9 (CBSE/NCERT) - Chapter 2 – Is Matter Around Us Pure – Questions and Answers/Notes/Worksheets, CBSE Class 9 - Chemistry – Chapter 2 - Is Matter Around Us Pure Practice Pages, Extra Question and Answer based on NCERT for Class 9th, Science Chemistry, CBSE Grade IX free Worksheets PDF Is Matter Around Us Pure exemplar question answer, NCERT Book question answer, Science Question bank on Is Matter Around Us Pure for ninth standard, Is Matter Around Us Pure, Smoke and fog both are aerosols. In what way are they different? You are given two samples of water labelled as ‘A’ and ‘B’. Sample ‘A’ boils at 100°C and sample ‘B’ boils at 102°C. Which sample of water will not freeze at 0°C? Comment. What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments? Classify the following into elements, compounds and mixtures. (a) Sodium (b) Soil (c) Sugar solution (d) Silver (e) Calcium carbonate (f) Tin (g) Silicon (h) Coal (i) Air (j) Soap (k) Methane (l) Carbon dioxide (m) Blood. List any two applications of crystallization. How Tyndall effect can be observed in the canopy of a dense forest? What effect is observed when sunlight passes through the canopy of a dense forest? Explain. Which method is used to separate two miscible liquids? What types of mixtures are separated by the technique of crystallisation? The ‘sea-water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment. List the two conditions essential for using distillation as a method for separation of the components from a mixture. Can we separate alcohol dissolved in water by using a separating funnel? If yes, then describe the procedure. If not, explain. Which of the tubes in Figure (a) and (b) will be more effective as a condenser in the distillation apparatus? Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed together. Will it be a pure substance or a mixture? Give reasons for the same. Give some examples of Tyndall effect observed in your surroundings? How would you confirm that a colourless liquid given to you is pure water? How do solution and gel differ from each other? Give one example for each. What is homogeneous mixture? Give examples.
-
Is Matter Around Us Pure
Q39. Smoke and fog both are aerosols. In what way are they different?
Ans. In smoke, the dispersed phase is solid and the dispersion medium is gas. In fog, the dispersed phase is liquid and the dispersion medium is gas.
Q40. You are given two samples of water labelled as ‘A’ and ‘B’. Sample ‘A’ boils at 100°C and sample ‘B’ boils at 102°C. Which sample of water will not freeze at 0°C? Comment
Ans. Sample ‘B’ boils at 102°C while the boiling point of pure water is 100°C. This means sample ‘B’ contains impurities. It will not freeze at 0°C.
Q41. What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments?
Ans. Pure gold is highly malleable and soft. When it is alloyed with copper or silver it becomes hard and strong and can be moulded into various shapes.
Q42. Classify the following into elements, compounds and mixtures.
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood
Ans. Elements – Sodium, Silver, Tin, Silicon
Compounds – Calcium Carbonate, Methane, Carbon Dioxide
Mixtures – Soil, Sugar Solution, Coal, Air, Soap, Blood
Q43. List any two applications of crystallization.
Ans. Applications
• Purification of salt that we get from sea water.
• Separation of crystals of alum (phitkari) from impure samples.
Q44. How Tyndall effect can be observed in the canopy of a dense forest?
Or
What effect is observed when sunlight passes through the canopy of a dense forest? Explain
Ans. Tyndall effect can be observed when sunlight passes through the canopy of a dense forest. In the forest, mist contains tiny droplets of water, which act as particles of colloid dispersed in air.
Q45. Which method is used to separate two miscible liquids?
Ans. Distillation is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
Q46. What types of mixtures are separated by the technique of crystallisation?
Ans. The crystallisation method is used to purify solids. For example, the salt we get from sea water can have many impurities in it. To remove these impurities, the process of crystallisation is used.
Q47. The ‘sea-water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment
Ans. Sea-water can be classified as a homogeneous mixture because it contains salts dissolved in water. It can be classified as a heterogeneous mixture also since it contains mud, sand and decayed parts of plants.
Q48. List the two conditions essential for using distillation as a method for separation of the components from a mixture.
Ans. It is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
Q49. Can we separate alcohol dissolved in water by using a separating funnel? If yes, then describe the procedure. If not, explain.
Ans. No, alcohol cannot be separated from water by using a separating funnel because alcohol is completely miscible in water. Only immiscible liquids can be separated using separating funnel.
Q50. Which of the tubes in Figure (a) and (b) will be more effective as a condenser in the distillation apparatus?
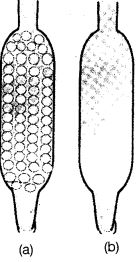
Ans. Figure (a) will be more effective condenser in the distillation apparatus because beads will provide more surface area for cooling of the vapours passing through it.
Q51. Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed together. Will it be a pure substance or a mixture? Give reasons for the same.
Ans. Sugar is a pure substance irrespective of the source of its preparation because it contains only one kind of pure matter and its composition is the same throughout.
Q52. Give some examples of Tyndall effect observed in your surroundings?
Ans. Examples of Tyndall effect:
1. When a fine beam of light enters a room through a small hole.
2. When sunlight passes through the canopy of a dense forest.
Q53. How would you confirm that a colourless liquid given to you is pure water?
Ans. Boiling point of water is 100°C at one atmosphere of pressure. We can confirm that a colourless liquid is pure by setting it to boil. If the liquid boils at 100°C, then it is pure water. This is because pure substances have fixed melting and boiling point.
Q54. How do solultion and gel differ from each other? Give one example for each.
Ans. A sol is a type of colloid in which solid is the dispersed phase and liquid is the dispersion medium. Examples include milk of magnesia, mud etc. A gel is a type of colloid in liquid is the dispersed phase and solid is the dispersed medium. Examples include jelly, cheese etc.
Q55. What is homogeneous mixture? Give examples.
Ans. A mixture in which the substances are completely mixed together and are indistinguishable from one another is called homogeneous mixture. Examples: Example: soda water, lemonade, air, petrol, sugar solution, salt solution, soft drinks etc.
-